Novotech Identifies Strategic Opportunities in Idiopathic Pulmonary Fibrosis Trials with Release of Global Market Insights
Novotech Identifies Strategic Opportunities in Idiopathic Pulmonary Fibrosis Trials with Release of Global Market Insights
SYDNEY--(BUSINESS WIRE)--Novotech, a globally recognized full-service clinical research organization (CRO) and scientific advisory partner for biotech and small- to mid-sized pharmaceutical companies seeking to advance drug development, has released an in-depth report on the global clinical trial landscape for Idiopathic Pulmonary Fibrosis (IPF). The report offers strategic insights and comprehensive analysis critical for biotech and pharmaceutical companies navigating the complexities of IPF clinical research and drug development.
Novotech, a globally recognized CRO has released an in-depth report on the global clinical trial landscape for Idiopathic Pulmonary Fibrosis.
Share
Advancing IPF Clinical Research: A Global Perspective
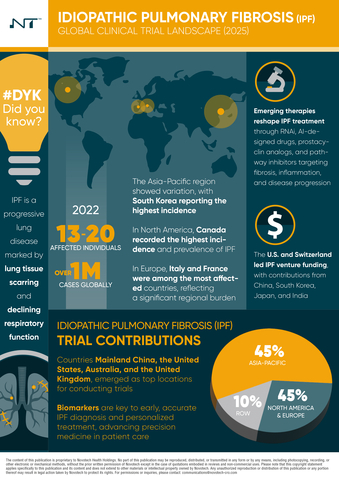
Idiopathic Pulmonary Fibrosis (IPF) is a chronic, progressive lung disease characterized by the scarring of lung tissue and a steady decline in respiratory function, most commonly affecting individuals between the ages of 60 and 70. Novotech’s latest report identifies more than 800 industry-sponsored IPF clinical trials launched globally since 2020, reflecting strong research momentum and a growing commitment across the biotech and pharmaceutical sectors to address the increasing burden of this serious condition.
Key insights:
- Global Clinical Trial Activity: The Asia-Pacific region accounts for the largest share of global IPF clinical trials at 44%, primarily driven by significant activity in Mainland China. North America follows with 23%, led by the United States, while Europe contributes 21%, with notable trial concentration in the United Kingdom and Germany.
- Emerging Therapeutic Innovations: The development pipeline is advancing with novel therapeutic approaches, including RNA interference (RNAi)-based therapies, PDE4 inhibitors, cell-based treatments, and monoclonal antibodies—signaling promising progress in IPF management and patient outcomes.
- Advancements in Trial Design and Biomarkers: Precision medicine and patient-centric trial methodologies, coupled with the integration of key biomarkers, are improving diagnostic accuracy, disease monitoring, and the assessment of therapeutic efficacy in IPF studies.
- Investment and Funding Landscape: Strong venture capital activity is fueling early- and mid-stage IPF drug development, particularly in the United States and Europe. Public-sector contributions, including funding from the EU Horizon 2020 program and the U.S. Department of Defense, are also instrumental in supporting innovation.
- Addressing Racial and Ethnic Disparities: The report highlights the critical need for greater diversity in IPF clinical trials and calls for equitable care strategies and targeted education to reduce treatment disparities across racial and ethnic populations.
Partnering with Novotech for IPF Clinical Trials
With extensive expertise in pulmonary and respiratory diseases, Novotech offers biotech and pharmaceutical partners an accelerated path to market. The company’s comprehensive capabilities include therapeutic expertise, regulatory guidance, and strategic clinical trial execution, supported by its expansive global footprint across Asia-Pacific, North America, and Europe.
About Novotech Novotech-CRO.com
Novotech is a globally recognized full-service clinical research organization (CRO) and scientific advisory company trusted by biotech and small- to mid-sized pharmaceutical companies to guide drug development at every phase.
With a global footprint that includes 30+ offices across the Asia-Pacific region, North America, and Europe and partnerships with 5,000+ trial sites, Novotech provides clients an accelerated path to bring life-changing therapies to market by providing access to key clinical trial destinations and diverse patient populations.
Through its client-centric service model, Novotech seamlessly integrates people, processes, and technologies to deliver customized solutions that accelerate the path to market for life-changing therapies. By adopting a true partnership approach, Novotech shares a steadfast commitment to client success, empowering innovation, and advancing healthcare worldwide.
Recipient of numerous industry accolades, including the Frost & Sullivan CRO Company of the Year award for 19 consecutive years, Novotech is recognized for its excellence in clinical trial execution and innovation. Its deep therapeutic and regulatory expertise, combined with local market insights, ensures streamlined clinical trials, optimized data analytics, and accelerated patient recruitment strategies.
Together with clients, Novotech transforms scientific advancements into therapies that improve global health outcomes, embodying a mission of driving innovation and delivering impactful results.
For more information or to speak to an expert team member, visit www.Novotech-CRO.com.
Contacts
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135